How Data Logging Improves Cannabis Recall Readiness
In the current regulatory landscape, a recall is not a matter of "if," but "when." The difference between a minor correction and a license-threatening event often reduces to a single variable: data accessibility.
When regulators demand a full production history, you generally have 24 hours to produce it. Operators relying on manual binders and fragmented spreadsheets face significant risk, especially given MarketWatch's analysis indicating that 88% of spreadsheets contain errors. Conversely, facilities with automated data logging can export accurate, verified information in minutes.
Data logging is a critical defense system, not just a compliance checkbox. By automatically capturing details at every production stage, manufacturers create a searchable, immutable digital trail.
This precision allows for targeted responses that limit recall scope and preserve brand reputation. This guide examines escalating recall risks, outlines required data points, and demonstrates how automated infrastructure turns raw numbers into your strongest liability shield.
Why Recall Readiness Is Now a Critical Cannabis Risk
Cannabis product recalls are increasing across regulated states as testing standards become more rigorous. The triggers are often microscopic but devastating: mold, yeast, Aspergillus, pesticide residues, or heavy metals. Even simple labeling errors or discrepancies in potency between the label and the lab result can force a product off the shelves.
The Financial and Reputational Costs of Recalls
The impact of a recall extends beyond the immediate loss of inventory. You must factor in legal fees, potential risk to your license, and operational disruption. However, the most expensive cost is often the damage to your brand. Retailers hesitate to restock products they view as a liability, and consumers rarely give a brand a second chance after a quality failure, and retailers hesitate to restock products they view as a liability. Implementing quality assurance robotics can significantly lower these risks by catching inconsistencies before they reach the shelf.
Meeting Evolving State and Federal Standards
State regulations now require faster response times and more granular documentation. Regulators are not just asking what you made; they want to know how you made it, who made it, and when it was made. With federal rescheduling on the horizon, FDA involvement is expected to bring even stricter standards that mirror the rigorous protocols seen in food and pharmaceutical manufacturing.
Will federal rescheduling automatically standardize recall protocols across all states?
Not immediately. While FDA involvement will likely establish a federal baseline for Good Manufacturing Practices (GMP) and recall procedures, individual states will likely maintain their own tracking systems (such as Metrc) and specific recall triggers for the foreseeable future. Manufacturers must be prepared to satisfy both potential federal oversight and existing state-level mandates simultaneously. Using robotics for cannabis compliance ensures you stay ahead of these shifting layers.
What Data Logging Really Means in Cannabis Manufacturing
There is often confusion about what "data logging" actually entails. Many operators assume that entering data into Metrc or BioTrack provides sufficient coverage.
That is a dangerous assumption.
State-mandated track-and-trace systems are primarily designed to monitor marijuana movement and generate tax revenue. They track the "what" and the "where" for tax purposes. They fail to capture the "how" for quality control. They do not record the specific temperature of an infusion reservoir, the calibration status of a kief-coating machine, or the exact environmental conditions during the curing process.
Comprehensive production data logging goes much deeper. It captures equipment settings, operator actions, environmental conditions, material inputs, and timestamps for every single action on the production floor.
This aligns with standards such as ASTM D8250-19, the Standard Practice for Applying a Hazard Analysis Critical Control Points (HACCP) System for Cannabis Consumable Products, which emphasizes the need to implement a system to prevent, control, or minimize manufacturing hazards.
The Difference Between Compliance and Preparedness
Meeting the minimum requirements for Metrc is compliance. It tells the state, "We tracked this plant from seed to sale."
Preparedness is operational resilience. Preparedness means you can find the exact processing parameters for a specific joint, explain why a deviation occurred, and identify every other product that might be affected within two hours. Compliance gets you a license, but preparedness protects your business when variables go wrong.
Do we need to log data for non-cannabis ingredients, such as terpenes or rolling papers?
Absolutely. Regulators can and do initiate recalls due to failing heavy-metal tests in rolling papers or contaminated botanical terpenes. A comprehensive data log must track the lot numbers of every input that comes into contact with the final product, not just the cannabis material itself.
How Data Logging Accelerates and Limits Cannabis Recalls
When a quality issue arises, speed is the most valuable asset. The difference between a minor operational hiccup and a headline-making disaster often comes down to how quickly you can isolate the problem. Here is how robust data logging transforms that response.
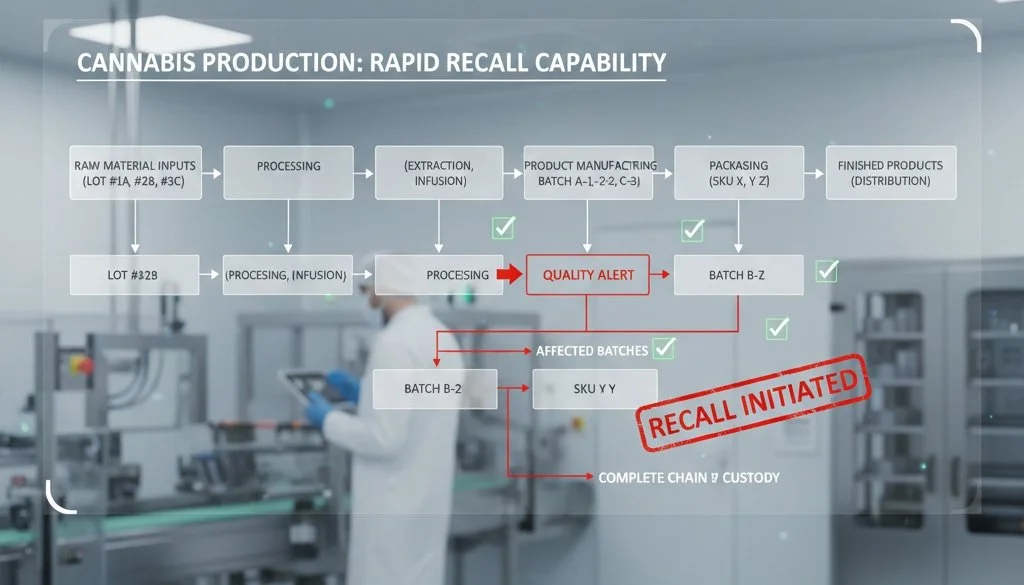
Pinpointing Affected Batches Quickly
Without granular data, operators are often forced to cast a wide net. If a contamination issue is detected but cannot be isolated to specific units, the facility must recall everything produced during that timeframe. This "any and all" approach devastates inventory and cash flow.
Complete batch records allow for surgical precision. If you can trace a contamination issue to a specific raw material lot used only on Tuesday morning between 9:00 AM and 11:00 AM, you can limit the recall to just those units. You save the rest of your stock. This methodology reflects the traceability principles outlined in Section 204 of the FDA’s Food Safety Modernization Act (FSMA), which mandates the maintenance of "Key Data Elements (KDEs)" associated with "Critical Tracking Events (CTEs)" to facilitate the faster identification and rapid removal of contaminated products from the market. Utilizing automated sorting systems during this phase ensures that only verified batches reach the consumer.
Documenting Chain of Custody
Cannabis products change hands multiple times. Every handoff from cultivation to processing to packaging to distribution must be logged.
When a recall hits, you need to know exactly where your products are. Chain of custody data supports clear communication with distributors and retailers. Instead of issuing a panic-inducing blanket warning, you can contact specific partners and tell them exactly which SKUs to pull. This protects downstream partners and limits the disruption to their business as well.
Supporting Root Cause Analysis
A recall is only resolved when the root cause is identified. Was it a machine malfunction? An operator error? A bad batch of distillate?
Production parameters logged at the time of the issue enable accurate diagnosis. If data shows a temperature spike in the infusion machinery during a specific run, you have identified the culprit. This insight allows for immediate corrective actions, preventing the issue from recurring. Without this data, operators are left guessing.
Satisfying Regulatory Documentation Demands
Auditors distinguish between real-time records and reconstructed summaries. They expect complete, contemporaneous records.
Digital logs with automatic timestamps are difficult to dispute. Auto-generated reports demonstrate that the facility is in control. When an operator can hand over a complete data package immediately upon request, it establishes credibility with regulators and shows the role of robotics in quality control.
Critical Data Points Every Cannabis Manufacturer Must Log
For operators looking to audit their current data practices, these are the non-negotiable data points that should be captured for every batch.
Essential Production-Level Data Points
Batch ID and Unique Product Identifiers: The foundational link for all other data.
Raw Material Lot Numbers: Including supplier info for flower, oil, and kief.
Equipment Used: Specific machine IDs and their calibration status at the time of the run.
Operator Identification: The specific personnel running the machine.
Production Settings: Temperature, pressure, dwell time, and dosing weight.
Environmental Conditions: Ambient humidity and room temperature during processing.
Critical Quality Control and Testing Records
COA Results: Linked directly to the specific batch records.
In-Process Testing: Moisture content or visual inspection results during production.
Non-Conformance Incidents: Records of any waste or rejected product and the reason why.
Packaging Verification: Confirmation that labels match the batch contents.
Distribution and Chain of Custody Logistics
Shipping Manifests: Detailed recipient information.
Storage Conditions: Temperature logs during transport for self-distributed product.
Delivery Confirmations: Proof of acceptance by the retailer.
What is the industry standard for data retention periods regarding production logs?
State regulations vary significantly. While some states have shorter windows, major markets like California require records to be retained for seven years (Cal. Code Regs. Tit. 4, § 15037). Best practice is to design your data retention policy to meet the strictest standard in any market you operate in, or indefinitely if digital storage costs are negligible.
Modern automated systems can capture many of these points without human intervention. This brings us to the role of automation.
How Automation Enables Consistent, Defensible Data Logging
Manual logging is inherently flawed. Operators experience fatigue. Steps are missed. Handwriting is illegible. Transcription errors occur.
That is where automated production equipment steps in as a compliance tool, not just a throughput solution. Automated systems like the Jiko infusion robot or Stardust kief coater capture data as a byproduct of their operation. They ensure consistency and accuracy that manual processes cannot match.
How Production Automation Captures Data Passively
Equipment sensors log parameters constantly. For example, automated infusion systems track injection pressure, reservoir temperature, and dispense volume for every unit, timestamped to the millisecond. This eliminates "best guess" documentation.
These digital logs can often integrate directly with batch tracking software. This removes the reliance on operators to copy numbers from a machine display to a clipboard and then to a computer. The data flows automatically, preserving the integrity of the record.
Connecting Automation to Recall Prevention
Automation does not just assist in cleanup; it aids in prevention.
Consistent automated dosing reduces the product variability that often triggers recalls for potency discrepancies. Documented process controls demonstrate due diligence to auditors. Furthermore, advanced systems can trigger automated alerts if parameters drift out of spec, allowing operators to stop the line and fix the issue before non-compliant products are manufactured.
Does automated data logging replace the need for a Quality Assurance (QA) manager?
No. Automation provides the tools for a QA manager to be effective, but it does not replace the role. The QA manager is still needed to interpret the data, oversee the mock recalls, audit the system for accuracy, and make the high-level decisions when a data anomaly is flagged by the automation.
How to Build a Recall-Ready Data Infrastructure
Building the infrastructure to support better data requires a systematic approach.
Conducting a Comprehensive Data Gap Analysis
Start by auditing existing documentation practices. Walk the production floor. Identify every point where a human is manually recording a critical number. These are risk points. Map the data flow from the moment the raw flower enters the facility to the moment a finished case leaves the dock.
Implementing Integrated Systems to Remove Silos
Data silos slow down response times. If lab results are in a PDF, production logs are on a clipboard, and inventory is in Metrc, efficient analysis is impossible.
Select equipment and software that communicate effectively. Prioritize platforms that offer API integrations or simple export functions to centralize records.
Standardizing Operating Procedures for Data Entry
Technology requires a process to function. Document data entry protocols clearly. Train staff not just on machine operation, but on documentation requirements. Assign specific responsibility for data accuracy.
Validating Readiness Through Regular Mock Recalls
Do not wait for a regulator to test the system. Conduct mock recalls.
Pick a random batch from three months ago. Time the team. Can they identify the raw material lots, the equipment used, and the current location of every unit within the state's required reporting window? If it takes four hours to locate the records, the process needs refinement. Use these drills to expose weaknesses and update SOPs.
Should we build a custom internal software solution or buy off-the-shelf tracking platforms?
For most mid-to-large operators, buying specialized manufacturing execution systems (MES) or ERPs that integrate with your hardware is superior to building custom software. Custom solutions often become "technical debt" that is hard to maintain, whereas established platforms update regularly to stay compliant with changing state regulations.
Common Data Logging Mistakes That Undermine Recall Readiness
Even well-intentioned operators fall into common traps. Avoid these specific pitfalls.
Relying Solely on Metrc: While necessary for tax compliance, treating Metrc as a quality management system creates a false sense of security. It leaves massive gaps in process history that defense strategies require.
Inconsistent Batch Numbering: If the cultivation team uses one numbering format and the processing team uses another, traceability breaks down. Standardize nomenclature across the entire facility.
Paper Records: Paper is easily lost, damaged, or misfiled. Digitizing records is the single most effective step to improve recall readiness.
Delayed Data Entry: Filling out logs at the end of the shift instead of in real-time creates gaps. Real-time capture is essential for accuracy.
The "Zero-Issue" Fallacy: Assuming a facility is safe because it has never had an issue often leads to skipped drills. Skipping mock recalls ensures that when a real event occurs, the team is testing the system for the first time during a crisis.
How does a "shadow system" of informal spreadsheets undermine recall readiness?
"Shadow systems" occur when staff keep their own private spreadsheets because the main system is too hard to use. In a recall, these siloed files create version-control conflicts, and management cannot verify which spreadsheet contains the final, accurate dataleading to incomplete or erroneous submissions. All data must be centralized in the official system of record to be useful during a crisis.
What Effective Data Logging Looks Like During a Recall
Consider the operational impact of proper data logging:
Rapid Identification: When a contamination alert arrives, the facility identifies the exact three batches affected out of the 50 produced that month within two hours.
Root Cause Isolation: Data reveals these batches shared a specific input from a single supplier, while equipment logs rule out internal contamination.
Regulatory Speed: The team generates a complete incident report with timestamped production data and submits it to regulators immediately.
Precision Recall: Distributors are notified with precise product codes, ensuring only the affected units are pulled.
Brand Protection: Reputation remains intact because the response was fast, professional, and transparent.
This is the standard professional operators aim for.
Can demonstrable recall readiness actually increase the valuation of a cannabis manufacturing brand?
Yes. Investors and acquirers view robust data infrastructure and recall readiness as indicators of operational maturity. A brand that can prove it has control over its supply chain is a lower-risk investment than one operating on "tribal knowledge" and paper logs.
Turn Data Logging into Your Strongest Recall Defense
Recall readiness is not a one-time project. It is an ongoing operational discipline that requires the right tools.
Automated production systems provide data logging as a built-in benefit, giving operators a competitive edge in both efficiency and compliance. Sorting Robotics equipment delivers real-time tracking alongside the throughput gains required to scale.
Evaluate current data practices. Identify the gaps. Then close them.
Protect your license and your brand. Visit SortingRobotics.com to implement the automated data infrastructure your compliance team demands.
Frequently Asked Questions
How does digital data logging reduce audit preparation time?
Digital logging transforms audit preparation from a days-long physical search into a near-instant process. By centralizing records, manufacturers can filter by batch ID and export comprehensive data packets in minutes, satisfying regulatory demands immediately.
How quickly should a cannabis company be able to respond to a recall?
Most state regulations expect a rapid response, often within 24 to 72 hours. Effective data logging allows manufacturers to identify affected products within hours rather than days, limiting recall scope and protecting consumers.
What is the difference between a voluntary and mandatory cannabis recall?
A mandatory recall is ordered by state regulators due to a confirmed threat to public health. A voluntary recall is initiated by the manufacturer upon discovering a potential issue. Robust data logging enables manufacturers to spot issues early and issue voluntary recalls, which often preserves brand trust better than forced regulatory action.
Can automation help with data logging for compliance?
Yes. Automated production equipment captures data as part of its normal operation, reducing reliance on manual entry and minimizing human error. Real-time dashboards and integrated software provide instant access to production records.
What are the most common causes of cannabis product recalls?
The most frequent causes include microbial contamination (mold, yeast, Aspergillus), pesticide residues, heavy metals, potency discrepancies, and labeling errors. Traceability data helps identify the source and limit the recall scope.
How often should a cannabis company conduct mock recalls?
Industry best practice recommends at least one mock recall per year, though quarterly exercises provide better preparedness. Mock recalls reveal documentation gaps and improve team response time.